electronic configuration of iodine in shells|Complete Electron Configuration for Iodine (I, I– ion) : Baguio Complete Electron Configuration for Iodine (I, I– ion) Options like Over 0.5 Goals and Over 1.5 Goals are also available, but the odds are much lower than for Over 2.5. It’s sometimes difficult to predict and requires some research, but Over 2.5 Goals is a popular bet for a good reason. It can be enjoyable to have a bet of this type on a game, especially if you’re watching it yourself.
electronic configuration of iodine in shells,Complete Electron Configuration for Iodine (I, I– ion)
Complete Electron Configuration for Iodine (I, I– ion)
Complete Electron Configuration for Iodine (I, I– ion)Iodine Electron Configuration: Everything You Need to Know
The total number of electrons in iodine is fifty-three. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in iodine in specific rules in different orbits and orbitals is called the electron configurationof iodine. The electron configuration of iodine is [Kr] . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit paAfter arranging the electrons, it is seen that the last shell of the iodine atom has seven electrons. Therefore, the valence electronsof iodine are seven. The elements . Tingnan ang higit pa
Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of iodine is 1s2 2s2 2p6 3s2 . Tingnan ang higit paMar 3, 2023 — How does the electronic configuration of Iodine explain its chemical properties? Iodine has 7 valence electrons, which makes it highly reactive and prone to .electronic configuration of iodine in shells119 rows — Mar 23, 2023 — Electron configuration chart of all Elements .
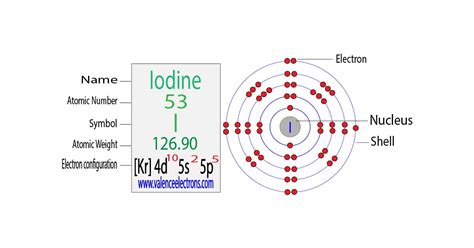
Electrons per shell. 2, 8, 18, 18, 7. Electron Configuration. [Kr] 4d 10 5s 2 5p 5. I. Kelp was the main source of natural iodine in the 18th and 19th centuries.Iodine has fifty-three protons and seventy-four neutrons in its nucleus, and fifty-three electrons in five shells. It is located in group seventeen, period five and block p of the .Dis 8, 2022 — The electronic configuration of an element is used to describe the arrangement of its electrons in its atomic orbitals. Let us discuss the energy levels that enclose an .Ene 30, 2023 — In the short notation, you place brackets around the preceding noble gas element followed by the valence shell electron configuration. The periodic table shows that kyrpton (Kr) is the .
Ago 2, 2018 — Schematic electronic configuration of iodine. The Kossel shell structure of iodine. Atomic spectrum. A representation of the atomic spectrum of iodine. Ionisation Energies and electron affinity. The .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .
Peb 1, 2021 — Iodine Electron Configuration: Iodine is a chemical element that has a symbol I. The atomic number of Iodine is 53. It is the heaviest of the stable halogens. It exists as a purple-black, lustrous, non-metallic .Mar 18, 2023 — The valency of the element is determined by electron configuration in the excited state. Here, bromine has three unpaired electrons. So in this case, the valency of bromine is 3. Bromide ion(Br –) .

Iodine atoms have 53 electrons and the electronic shell structure is [2, 8, 18, 18, 7] with Atomic Term Symbol (Quantum Numbers) 2 P 3/2. Atomic Number: 53: . Complete ground state electronic configuration for the .
Ago 14, 2020 — The electron configurations and orbital diagrams of these four elements are: Figure \(\PageIndex{5}\): Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in a .Ago 22, 2024 — Hint: From the electronic configuration of Iodine, we can know about the valence electrons present and thereby finding the number of shells in which valence electrons of iodine lie. Complete step by step answer: Iodine, I, is located in period 5 and group 17 of the periodic table. The Atomic Number of element Iodine is $53$. The .
Write the subshell electron configuration (i.e. 1s^2 2s^2, etc.) for the Si14 atom and identify which are valence (outer shell) electrons and determine how many valence electrons there are. Write the electron configuration you would expect for iodine (Z = 53). Use a noble gas core. How do electron configurations correspond to the periodic table?Nob 13, 2020 — Iodine is a chemical element with atomic number 53 which means there are 53 protons and 53 electrons in the atomic structure.The chemical symbol for Iodine is I. Electron Configuration and Oxidation States of Iodine. Electron configuration of Iodine is [Kr] 4d10 5s2 5p5. Possible oxidation states are +1,5,7/-1. Electron .

Iodine; Astatine; The term halogen has been derived from the Greek language which means salt producers, as halo means ‘salts’ and genes mean ‘born’. . Electronic configuration can simply be defined as the arrangement of electrons of an atom in its orbitals. Studying the electronic configuration of an atom or a molecule helps one .Mar 16, 2023 — Therefore, the electron configuration of sodium is 1s2 2s2 2p6 3s1. The atomic number of sodium is 11, which means its atom has eleven electrons. . Sodium donates an electron of the last shell to form bonds and turns into a . (Br), iodine(I), and astatine(At). The s-block element is sodium. Sodium reacts with halogen to form halide .Hul 20, 2023 — A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. An atom may give, take, or share electrons with another atom to achieve a full valence shell, the most stable electron configuration.Electronic configuration of the Iodine atom in ascending order of the levels: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 5 Reduced electronic configuration I: [Kr] 4d 10 5s 2 5p 5. Below is the electronic diagram of the Iodine atom Distribution of electrons over energy levels in the I atomComplete Electron Configuration for Iodine (I, I– ion)In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus.The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the .Note that these electron configurations are given for neutral atoms in the gas phase, which are not the same as the electron configurations for the same atoms in chemical environments. In many cases, multiple configurations are within a small range of energies and the irregularities shown below do not necessarily have a clear relation to .
The principal quantum shells increase in energy with increasing principal quantum number. E.g. n = 4 is higher in energy than n = 2 The subshells increase in energy as follows: s < p < d < f. The only exception to these rules is the 3d orbital which has slightly higher energy than the 4s orbital; Because of this, the 4s orbital is filled before the 3d orbitalMay 17, 2023 — The 53rd element of the periodic table is iodine. The element of group 17 is iodine and its symbol is ‘I’. The valence electrons are the total number of electrons in the last orbit (shell).. The total number of electrons in the last shell after the electron configuration of iodine is called the valence electrons of iodine. The last shell of .
Hun 27, 2024 — Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Writing the electronic structure of an element from hydrogen to krypton. Use the Periodic Table to find the atomic number, and hence number of electrons. Fill up orbitals in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p - until you run out of electrons. The 3d is the awkward one - remember that specially. . Iodine is in group 7 and so has 7 outer .Ago 2, 2018 — Iodine atoms have 53 electrons and the shell structure is 2.8.18.18.7. The ground state electron configuration of ground state gaseous neutral iodine is [Kr].4d 10.5s 2.5p 5 and the term symbol is 2 P 3/2.
electronic configuration of iodine in shells|Complete Electron Configuration for Iodine (I, I– ion)
PH0 · WebElements Periodic Table » Iodine » properties of
PH1 · Iodine Electronic Configuration and Distribution in Shells
PH2 · Iodine Electronic Configuration and Distribution in Shells
PH3 · Iodine Electron Configuration: Everything You Need to Know
PH4 · Iodine Electron Configuration (I) with Orbital Diagram
PH5 · Iodine (I)
PH6 · Iodine
PH7 · Electron Configuration Chart of All Elements (Full Chart)
PH8 · Electron Configuration
PH9 · Complete Electron Configuration for Iodine (I, I– ion)